This is the continuation of the previous notes. In this post, I am going to talk point number 4 to 6.
4. HOW THE STRUCTURE OF A COMPOUND IS REPRESENTED
The structure of a compound can be presented in 4 types: Lewis structures, Kekule structures, condensed structures, and skeletal structures.
In Lewis structures, we can see the bonds, lone pair electrons, and formal charge. The bonds do not tell the bond angles in the actual molecules. Lone pair electrons are the valence electrons which are not used in the bonding and are also usually called as nonbonding electrons, lone-pair electrons, and lone pairs. While the formal charge is the charge contained in an atom. We can know how much is the charge by following the formulation below.
(1)
Based on the formulation, could you guess how much is there any atoms that have a formal charge? If yes, please put them in each atom.
When a formal charge is equipped in an atom, each atom has a specific name.
Next, let's study how to draw Lewis structure from a molecule. Could you draw the Lewis structure of HNO2?
Firstly, let's determine the total valence electrons for HNO2 (1+5+12 = 18 valence electrons). Secondly, draw the possible bonding.
We can see, there are 3 single bonds that in total have 6 electrons. Finally, we can draw the lone pairs by subtracting the total valence electrons with the total electrons in bonding, 18 - 6 = 12. It means that there might be 12 lone pairs. We have to make sure by putting the lone pairs in the possible atom and calculate the formal charge that should be zero. If it's not zero, consider double bonds or rearrange the structure.
Let's check the formal charge for N atom, 5 - (2+2) = 1, while for O atom with red color, 6 - (6+1) = -1. Meanwhile, it should not have a formal charge for both N atom and O atom. So, the structure of the molecule should be change. In this case, let's consider the double bond by using one lone pairs from O atom.
The formal charge for N atom, 5 - (2+3) = 0, while for O atom 6 - (4+2) = 0. By this calculation, now we can have 0 for both atoms. It means that we have successfully drawn the Lewis structure of HNO2.
After studying the Lewis structures, let's move to Kekule structures. Kekule structures have the same structure with Lewis except this structure omits the lone pairs.
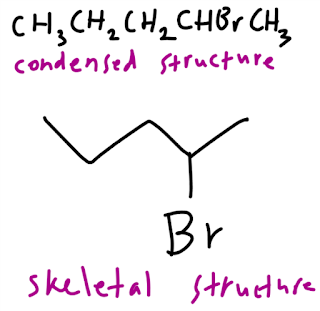
The third, condensed structures. Condensed structures omit the line for the bond except for some important functional groups. Look the example below.
The last, skeletal structures. This structure focuses only on important functional groups while the atom carbon with hydrogen in its bonding is not shown. It is just drawn with a line like the example below.
5. ATOMIC ORBITALS
Now, we are going to talk about the location of the electron. Based on Heisenberg uncertainty principle, the precise location of the electron cannot be determined, we only can describe its probable location. Atomic orbital is a three-dimensional region (volume of space) around the nucleus where probably we can find the electron.
There are 4 atomic orbitals: s, p, d, f, yet in organic chemistry, mostly we only use s and p atomic orbitals since in organic chemistry we use carbon atom mostly. In s atomic orbitals, there are 1s, 2s, and higher. The difference is the location, 1s atomic orbital is located close to the nucleus, while 2s is located farther. In atomic orbitals, we should know the term "node", node is the area where we can not find any electrons.
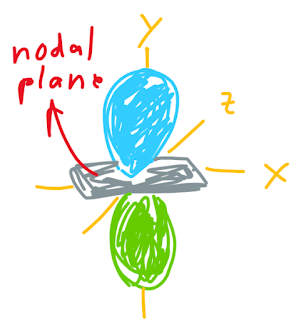
p atomic orbitals have two lobes, each lobe has different phase. We can differ it by different color. The phase doesn't indicate the difference in charge. The node in p atomic orbitals is called as nodal plane. The same like node in s atomic orbitals, there is zero probability we can find electrons in the nodal plane.
6. AN INTRODUCTION TO MOLECULAR ORBITAL THEORY
An atomic orbital surrounds an atom, while a molecular orbital surrounds a molecule. The molecule orbitals may allow you to see the probability in volume of space of an electron when an atom binds to another atom in order to complete it into an octet. In molecular orbital theory, at least there are 4 terms that we should know: sigma bond, bonding molecular orbitals, antibonding molecular orbitals, and pi bond.
Sigma bond is a covalent bond when two s orbitals overlap. The bond shape is cylindrical and the electron is symmetrically distributed that is connecting two nuclei from 2 s orbitals.
What makes the covalent bond formed is the attraction of two negatively charged electrons and two positively charged nuclei. Therefore, at the time when the bond formed, the energy decreases. The more the orbitals overlap, the more the energy decreases. However, there is a limit of how close the orbital can overlap. At the time when two nuclei are so close and begin to repel each other is the limit of the distance. This distance of the bond is called as the bond length and this distance is the maximum stability.
If the bond wants to dissociate, it requires the same energy with the energy released when the bond formed. In other words, forming the bond will release energy, while breaking the bond will require energy that the number of energy required is precisely the same as the energy released when the bond formed.
When we are talking about molecular orbitals, actually the number of molecular orbitals formed is the total number of atomic orbitals that bind each other. For example, if two atomic orbitals of hydrogen bind, it means that two molecular orbitals are also formed. Before this, we only talked about 1 molecular orbital, the other remaining molecular orbital is the antibonding molecular orbital.
How come we have 2 types of molecular orbital? As the electron acts as wave-like properties, it does have two manners: additive manner and destructive manner. So, the additive manner in this molecular orbitals we call bonding molecular orbital while the destructive manner we all antibonding molecular orbital (sigma star bonding).
The bonding molecular orbital formed when waves with the same phase overlap constructively, while the antibonding molecular orbital formed when waves with different phase overlap destructively.
In bonding molecular orbital, two same phases reinforce each other to form bonding. While in the antibonding molecular orbital, two different phases cancel each other and no bonding formed.
If we are talking about energy, at the time when two atomic orbitals overlap, one is lower in energy (bonding molecular orbital) and one is higher in energy (antibonding molecular orbital).
How to assign electrons in the molecular orbitals is the same as in the atomic molecular orbital. The electron will fill the available orbital with the lowest energy which is the bonding molecular orbital. From the picture above, we can see that bonding molecular orbital is lower in energy than the atomic molecular orbital, thus more stable. Another rule, no more than 2 electrons can occupy one orbital. Therefore, we can see that H2 does exist. Two electrons from two atomic orbitals occupy the bonding molecular orbital. While no electron in the antibonding molecular orbital. How about He2? Does it exist?
It does not exist because the total electrons are 4, means that 2 electrons in the bonding molecular orbital and the other 2 remaining electrons will fill the antibonding electrons which cancel the bonding form.
Let's talk about pi bond, what is the difference between sigma bond and pi bond? Sigma bond is the bond formed when two s atomic orbitals overlap, while pi bond is the bond formed when two p atomic orbitals overlap. The same like sigma bond, pi bond also has bonding and antibonding molecular orbital. Bonding molecular orbital form when two in-phase (green lobe with green lobe, blue lobe with blue lobe) atomic orbitals side-to-side overlap. Meanwhile, antibonding molecular orbital formed when two out-of-phase (green lobe with blue lobe) atomic orbitals side-to-side overlap.
Until this, do you still remember Lewis structure? From Lewis structure, we can see the overview of a molecule about how much lone pair electrons and how much bond formed in a molecule. However, the shape of the molecule can not be studied by Lewis structure. If we want to see the first glance of the molecule shape, we can use VSEPR (Valence-shell electron-pair repulsion (VSEPR) model. This model can predict the geometry of the molecule because not only electron in bonding molecular orbital that matters in the molecular shape, the lone pairs also matters. Remember that lone pairs are also negatively charged so when there is a bonding, they will repel each other and positioned as far as possible. That's why lone pairs also influence the shape of the molecule.
Now, we are going to talk about the location of the electron. Based on Heisenberg uncertainty principle, the precise location of the electron cannot be determined, we only can describe its probable location. Atomic orbital is a three-dimensional region (volume of space) around the nucleus where probably we can find the electron.
There are 4 atomic orbitals: s, p, d, f, yet in organic chemistry, mostly we only use s and p atomic orbitals since in organic chemistry we use carbon atom mostly. In s atomic orbitals, there are 1s, 2s, and higher. The difference is the location, 1s atomic orbital is located close to the nucleus, while 2s is located farther. In atomic orbitals, we should know the term "node", node is the area where we can not find any electrons.
6. AN INTRODUCTION TO MOLECULAR ORBITAL THEORY
An atomic orbital surrounds an atom, while a molecular orbital surrounds a molecule. The molecule orbitals may allow you to see the probability in volume of space of an electron when an atom binds to another atom in order to complete it into an octet. In molecular orbital theory, at least there are 4 terms that we should know: sigma bond, bonding molecular orbitals, antibonding molecular orbitals, and pi bond.
Sigma bond is a covalent bond when two s orbitals overlap. The bond shape is cylindrical and the electron is symmetrically distributed that is connecting two nuclei from 2 s orbitals.
What makes the covalent bond formed is the attraction of two negatively charged electrons and two positively charged nuclei. Therefore, at the time when the bond formed, the energy decreases. The more the orbitals overlap, the more the energy decreases. However, there is a limit of how close the orbital can overlap. At the time when two nuclei are so close and begin to repel each other is the limit of the distance. This distance of the bond is called as the bond length and this distance is the maximum stability.
If the bond wants to dissociate, it requires the same energy with the energy released when the bond formed. In other words, forming the bond will release energy, while breaking the bond will require energy that the number of energy required is precisely the same as the energy released when the bond formed.
When we are talking about molecular orbitals, actually the number of molecular orbitals formed is the total number of atomic orbitals that bind each other. For example, if two atomic orbitals of hydrogen bind, it means that two molecular orbitals are also formed. Before this, we only talked about 1 molecular orbital, the other remaining molecular orbital is the antibonding molecular orbital.
How come we have 2 types of molecular orbital? As the electron acts as wave-like properties, it does have two manners: additive manner and destructive manner. So, the additive manner in this molecular orbitals we call bonding molecular orbital while the destructive manner we all antibonding molecular orbital (sigma star bonding).
The bonding molecular orbital formed when waves with the same phase overlap constructively, while the antibonding molecular orbital formed when waves with different phase overlap destructively.
In bonding molecular orbital, two same phases reinforce each other to form bonding. While in the antibonding molecular orbital, two different phases cancel each other and no bonding formed.
If we are talking about energy, at the time when two atomic orbitals overlap, one is lower in energy (bonding molecular orbital) and one is higher in energy (antibonding molecular orbital).
How to assign electrons in the molecular orbitals is the same as in the atomic molecular orbital. The electron will fill the available orbital with the lowest energy which is the bonding molecular orbital. From the picture above, we can see that bonding molecular orbital is lower in energy than the atomic molecular orbital, thus more stable. Another rule, no more than 2 electrons can occupy one orbital. Therefore, we can see that H2 does exist. Two electrons from two atomic orbitals occupy the bonding molecular orbital. While no electron in the antibonding molecular orbital. How about He2? Does it exist?
It does not exist because the total electrons are 4, means that 2 electrons in the bonding molecular orbital and the other 2 remaining electrons will fill the antibonding electrons which cancel the bonding form.
Let's talk about pi bond, what is the difference between sigma bond and pi bond? Sigma bond is the bond formed when two s atomic orbitals overlap, while pi bond is the bond formed when two p atomic orbitals overlap. The same like sigma bond, pi bond also has bonding and antibonding molecular orbital. Bonding molecular orbital form when two in-phase (green lobe with green lobe, blue lobe with blue lobe) atomic orbitals side-to-side overlap. Meanwhile, antibonding molecular orbital formed when two out-of-phase (green lobe with blue lobe) atomic orbitals side-to-side overlap.
Until this, do you still remember Lewis structure? From Lewis structure, we can see the overview of a molecule about how much lone pair electrons and how much bond formed in a molecule. However, the shape of the molecule can not be studied by Lewis structure. If we want to see the first glance of the molecule shape, we can use VSEPR (Valence-shell electron-pair repulsion (VSEPR) model. This model can predict the geometry of the molecule because not only electron in bonding molecular orbital that matters in the molecular shape, the lone pairs also matters. Remember that lone pairs are also negatively charged so when there is a bonding, they will repel each other and positioned as far as possible. That's why lone pairs also influence the shape of the molecule.
That's all for today. I apologize if there is a mistake. I hope you can get something from here. See you on another posting! Thanks for visiting!
Reference:
Bruice, P. Y. 2017. Organic Chemistry Eighth Edition. England: Pearson Education Limited.
Reference:
Bruice, P. Y. 2017. Organic Chemistry Eighth Edition. England: Pearson Education Limited.